Cracking the code of hydrogen evolution on platinum
GA, UNITED STATES, October 14, 2025 /EINPresswire.com/ -- Hydrogen evolution and oxidation reactions (HER/HOR) on platinum play a central role in clean energy technologies, yet their mechanisms in alkaline environments remain poorly understood. This study uncovers how alkali metal cations and hydroxide anions interact within the electrical double layer (EDL) to shape catalytic performance. By systematically varying sodium and hydroxide concentrations, the researchers revealed that EDL thickness and interfacial electric fields directly determine HER/HOR activity. Thinner EDLs with stronger electric fields facilitate water dissociation and hydrogen recombination, accelerating reaction rates. Conversely, excessive ion concentrations or disrupted hydration structures weaken electric fields and hinder kinetics. These findings highlight EDL engineering as a critical pathway to enhance electrochemical hydrogen systems.
Hydrogen reactions on platinum have long intrigued scientists for their apparent simplicity yet surprisingly sluggish kinetics in alkaline media. The challenge lies in understanding how cations, anions, and water molecules arrange and interact at the electrode surface. Traditional explanations, such as hydrogen binding energy or bifunctional mechanisms, often fail to capture the full complexity. Recent studies suggested cations can either stabilize reaction intermediates or block catalytic sites depending on their concentration, but no unified picture has emerged. Based on these challenges, there is a need to conduct systematic research that isolates the effects of different ionic species and clarifies their role in Hydrogen evolution and oxidation reactions (HER/HOR) kinetics.
A research team from the University of California, Los Angeles, has published new findings on June 9, 2025, in Precision Chemistry. The study investigates how sodium and hydroxide ions reshape the electrical double layer on platinum catalysts and impact alkaline hydrogen reactions. By designing 28 electrolytes with independently varied ion concentrations, the researchers uncovered a clear correlation between electrical double layer (EDL) thickness, interfacial electric fields, and HER/HOR activity, providing fresh insights into electrochemical interface design.
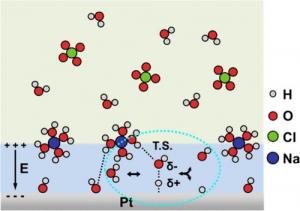
The team designed a systematic set of electrolytes, independently varying sodium (Na⁺) and hydroxide (OH⁻) concentrations between 0.001 and 1.0 M, to decouple their effects on platinum-catalyzed hydrogen reactions. Results showed that HER/HOR activity consistently tracked with EDL thickness: thinner EDLs produced stronger electric fields and higher reaction rates. At moderate Na⁺ or OH⁻ levels (<0.1 M), EDLs compressed, interfacial water molecules became more polarized, and the Volmer step—the rate-determining stage involving water dissociation or Had/OH⁻ recombination—accelerated. However, beyond 0.1 M Na⁺, unexpected suppression occurred. The team attributed this to ion-pair formation at the outer Helmholtz plane, which weakened electric fields and rigidified water structures. At extremely high alkalinity (pH 14), partial dehydration of sodium ions further disrupted interfacial water, reducing efficiency despite stronger fields. Electrochemical impedance spectroscopy confirmed these trends, linking higher capacitance with thinner EDLs and enhanced kinetics, except under extreme conditions. Together, these findings establish EDL thickness and surface electric field as critical descriptors of hydrogen reactivity, offering a physical model that reconciles earlier fragmented observations.
According to the paper, hydrogen catalysis is not governed solely by hydrogen binding energy, but by the delicate balance of ion concentrations, electric fields, and interfacial water structure. The counterintuitive effects—where too many ions suppress rather than enhance activity—underscore the complexity of the electrochemical interface. By identifying EDL thickness as a central descriptor, a unifying framework is provided that can guide the rational tuning of electrolytes and catalysts for next-generation hydrogen technologies.
This work offers practical design principles for hydrogen-based energy systems, including alkaline water electrolyzers and anion exchange membrane fuel cells. By tuning ion concentrations to achieve optimal EDL thickness, engineers can enhance reaction rates and efficiency without solely relying on costly catalyst redesign. The study also suggests that moderate electrolyte concentrations yield the best balance between electric field strength and interfacial water organization. Looking ahead, the approach can be extended to other cations such as potassium or cesium, which may display distinct hydration effects. Such insights pave the way toward more efficient and durable electrochemical platforms for sustainable hydrogen production and utilization.
References
DOI
10.1021/prechem.5c00019
Original Source URL
https://doi.org/10.1021/prechem.5c00019
Funding Information
Y.H. acknowledges support under the National Science Foundation award 2404462.
Lucy Wang
BioDesign Research
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.