Duchenne Muscular Dystrophy (DMD) Therapeutics Market to Hit US$6.64B by 2033, Industry Developments, Future Growth
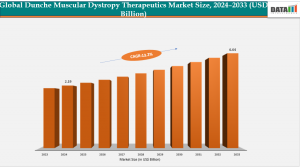
Explosive Growth in Duchenne Muscular Dystrophy Treatment Market: US$2.19B → US$6.64B by 2033
USA Duchenne Muscular Dystrophy (DMD) Therapeutics Market 2025-2033: Strong 13.2% CAGR Driving Market from US$2.19B to US$6.64B By 2033”
AUSTIN, TX, UNITED STATES, October 13, 2025 /EINPresswire.com/ -- Market Size and Forecast— DataM Intelligence 4Market Research LLP
According to the DataM Intelligence: The global Duchenne Muscular Dystrophy (DMD) Therapeutics Market size was valued as US$ 2.19 billion in 2024 and is expected to reach US$ 6.64 billion by 2033, growing at a CAGR of 13.2% during the forecast period 2025-2033.
Get a Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):–https://www.datamintelligence.com/download-sample/global-dunche-muscular-dystrophy-therapeutics-market
Key Highlights – Duchenne Muscular Dystrophy Therapeutics Market
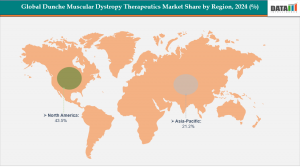
North America leads the market, capturing the largest revenue share of 43.5% in 2024.
Asia Pacific is the fastest-growing region, projected to expand at a CAGR of 8.1% over the forecast period.
By therapeutic type, the molecular-based segment held the largest revenue share of 45.1% in 2024.
Key players in the market include Sarepta Therapeutics, PTC Therapeutics, Nippon Shinyaku (NS Pharma), ITF Therapeutics, and Catalyst Pharmaceuticals, among others.
• The Duchenne Muscular Dystrophy therapeutics market has witnessed significant advancements in gene and molecular therapies over the past few years. One notable development is the approval of mutation-targeted exon-skipping therapies, such as Exondys 51, Vyondys 53, and Amondys 45 by Sarepta Therapeutics, and Viltepso by Nippon Shinyaku. These therapies are designed to bypass specific genetic mutations in the DMD gene, allowing for partial restoration of functional dystrophin protein. .
• In addition, gene therapy and cell-based approaches are rapidly advancing, representing a transformative shift in DMD treatment. Sarepta’s Elevidys (SRP-9001) and Capricor’s Deramiocel (CAP-1002) are at the forefront, targeting underlying genetic defects and offering the potential for long-term disease modification. Regulatory agencies, including the FDA and EMA, are increasingly granting accelerated approvals, orphan drug designations, and breakthrough therapy designations, facilitating faster market access for these innovative treatments.
Key Industry Developments
In September 2025, Capricor Therapeutics, a biotech company developing cell- and exosome-based therapies for rare diseases, provided a regulatory update on its Biologics License Application (BLA) for Deramiocel, an investigational cell therapy for Duchenne Muscular Dystrophy (DMD). This followed a Type A meeting with the FDA after receiving a Complete Response Letter (CRL) in July 2025.
Meanwhile, the high cost of DMD treatments remains a major barrier. Sarepta’s Elevidys is priced around $3.2 million per patient, while exon-skipping therapies like Exondys 51 and Viltepso range from $300,000 to $600,000 annually. Such steep prices strain healthcare systems and families, particularly in regions with limited insurance coverage, limiting equitable access and broader adoption despite significant scientific progress.
Major Companies:
Top companies in the global dunche muscular dystrophy therapeutics market include sarepta therapeutics, ptc therapeutics, nippon Shinyaku (NS Pharma), ITF THERAPEUTICS, Catalyst Pharmaceuticals and among others.
Get Customization in the report as per your requirements:- https://www.datamintelligence.com/customize/global-dunche-muscular-dystrophy-therapeutics-market
Duchenne Muscular Dystrophy (DMD) Treatment Market: Growth & Challenges
• Rising innovations in DMD treatment are driving market growth. Gene therapies, like Elevidys, target the root cause absence of dystrophin slowing disease progression and showing sustained functional benefits in both ambulatory and non-ambulatory patients. Exon-skipping therapies, such as Exondys 51 and Vyondys 53, help produce functional dystrophin, improving motor outcomes and expanding treatment options.
• However, high drug costs remain a major barrier. Gene therapy Elevidys is priced around $3.2M per patient, while exon-skipping drugs exceed $300K annually, limiting access, especially in low- and middle-income regions. Despite groundbreaking advances, affordability challenges are slowing market adoption and expansion.
Recent Developments:
• In March 2024, Italfarmaco S.p.A. announced the U.S. Food and Drug Administration (FDA) approval of Duvyzat (givinostat), a novel histone deacetylase (HDAC) inhibitor, for the treatment of patients 6 years or older with Duchenne muscular dystrophy (DMD), a rare X-linked progressive and life-limiting neuromuscular condition with symptoms from early childhood.
Duchenne Muscular Dystrophy (DMD) Therapeutics Market – Regional Insights
North America: Leading the global DMD therapeutics market with 43.5% share in 2024, driven by strong FDA support, high healthcare spending, and early adoption of advanced therapies. Key players like Sarepta, PTC, and Capricor are advancing gene and molecular-based treatments. For example, in August 2025, the FDA granted Breakthrough Therapy designation to delpacibart zotadirsen (del-zota), an innovative RNA-based therapy targeting exon 44 skipping.
Europe: Holding 34.5% of the market, Europe benefits from orphan drug approvals, robust healthcare policies, and expanding clinical research. Germany is a key market, with strong funding and adoption of exon-skipping and gene therapies. In January 2024, Santhera Pharmaceuticals launched AGAMREE (vamorolone) for DMD patients aged 4+, regardless of mutation or ambulatory status.
Asia-Pacific: The fastest-growing region with a CAGR of 8.1%, driven by rising healthcare investment, improved diagnosis, and collaborations with global biotech firms. Japan is a leader in DMD innovation, early adoption of exon-skipping therapies, and strong government support. In September 2025, DYNE-251 received Orphan Drug designation in Japan and is undergoing Phase 1/2 clinical trials targeting exon 51 skipping.
Market Segmentation
Molecular-based therapies lead DMD market with 45.1% share
Intravenous administration dominates DMD market with 46.1% share
By Therapeutic Type: (Molecular-Based, Steroidal Therapy, NSAIDs, Others)
By Mutation Type: (Exon 51 Skipping, Exon 53 Skipping, Exon 45 Skipping, Others)
By Route of Administration: (Intravenous, Subcutaneous, Others)
By Distribution Channel: (Hospital Pharmacies, Specialty Pharmacies)
Buy Now & Unlock 360° Market Intelligence:- https://www.datamintelligence.com/buy-now-page?report=global-dunche-muscular-dystrophy-therapeutics-market
Analyst Concludes: The Duchenne Muscular Dystrophy (DMD) therapeutics market as highly promising, driven by innovative gene and molecular therapies and increasing regulatory support for rare disease treatments. They highlight that companies like Sarepta, PTC Therapeutics, and Italfarmaco are setting the pace with approved and late-stage therapies, while ongoing advancements in gene editing, RNA therapeutics, and cell therapy are expected to expand patient access and improve clinical outcomes.
Related Reports:
Duchenne Muscular Dystrophy Treatment Market
North America Duchenne Muscular Dystrophy Treatment Market
About Us:
DataM Intelligence 4Market Research is a market intelligence platform that gives access to syndicated, customized reports and consulting to its clients in one place. As a firm with rich experience in research and consulting across multiple domains, we are a one-stop solution that will cater to the needs of clients in key business areas. DataM Intelligence has an online platform whose coverage includes industries such as chemicals and materials, agriculture, health care services, animal feed, and food & beverages, among others.
Our platform has Insights on markets that uncover the latest market research data that are distinct from the competition. With coverage across 10 major industries in the marketplace research, dataM Intelligence benefits thousands of companies by helping them take their innovations early to the market, and by providing a complete view of the market with statistical forecasts. Our strategy-centric framework and value-added services will let individuals and corporates with ease of access and custom personalization to research and markets.
Author: Gundreddy Gopinadh is a healthcare research analyst with over a decade of experience in medical devices, pharmaceuticals, biotechnology, and in-vitro diagnostics (IVD). He specializes in delivering actionable insights through custom market research projects, helping organizations analyze trends, assess competitive landscapes, and uncover growth opportunities. His expertise has guided healthcare companies in product development, strategic planning, and market expansion, positioning him as a trusted contributor to data-driven innovation in the global healthcare industry.
Sai Kiran
DataM Intelligence 4Market Research LLP
+1 877-441-4866
sai.k@datamintelligence.com
Visit us on social media:
LinkedIn
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.